
The Galaxy Automated Nucleic Acid Purification and qPCR System consists of intelligent cassette (iCassette) and Galaxy series automated fully enclosed qPCR instrument (Galaxy Nano, Galaxy Lite or Galaxy Pro). The system can intelligently complete the whole process of nucleic acid extraction, purification, qPCR amplification and analysis functions. It truly realizes integrated and fully enclosed qPCR detection.
Features
Intelligent
Users only need to load samples (blood, serum, essential substances, urine, tissues, and etc.) into the system. System can recognize sample type and protocol, then receive multiplex qPCR result automatically. Achieve "sample in, results out", reduce manual operation steps.
Integrated
Performs nucleic acid extraction, amplification, and detection all in one instrument. No need for supporting equipments, such as a centrifuge, biosafety cabinet, refrigerator, and etc, saving the overall equipment investment.
Fully Enclosed
Pollution free, biosafety guaranteed, no need for PCR lab. Our product saves lab space and lab construction investment.
Multiplex qPCR Detection
7 color fluorescent channels for multiplex detection.
Rapid Detection
1-2 hours to get results, shorten turn-around time.
Sample in Results Out
Galaxy Automated Nucleic Acid Purification and qPCR System
After samples are loaded into the iCassette, the iCassette can be placed on any model of Galaxy instruments to fully auto-complete the whole process of nucleic acid extraction, amplification, qPCR detection and result analysis.
| Galaxy Pro | Galaxy Lite | Galaxy Nano | ||
| Intelligent | Automatic nucleic acid extraction and purification | Yes | ||
| Automatic transfer of nucleic acid to the amplification area | Yes | |||
| Automatic protocol programming | Yes | |||
| Automatic qPCR results analysis | Yes | |||
| Heat ramping | ≧ 4.2℃/s | |||
| Fluorescence channel | 7 colors:Fam, Hex, Tamra, Rox, Cy5, Cy5.5, Amca | |||
| Multiplex melting curve analysis | Supported up to 54 plex | Supported up to 18 plex | ||
| Throughput | 1-12 samples | 1-6 samples | 1 sample | |
| Fluorescent linear | R≥0.99 | |||
| Precision | CV≤ 3% | |||
| Size | 550*480*400mm | 327*405*473mm | 175*285*435mm | |
The Galaxy Pro can be upgraded to run 96 samples in parallel, 16 protocols asynchronous The Galaxy Lite can be upgraded to run 24 samples in parallel, 4 protocols asynchronous.
Igenesis has a strong reagent development and production capacity. Our high-quality ready-for-use multiplex detection reagent products help clinical hospitals, CDCs and various medical research units to carry out various detection projects.
| Classification | Item No. | Product Name | |
| SARS-2-CoV(Covid-19) |  |
106-0050-01 | SARS-2-CoV(Covid-19) nucleic acid detection cartridge (Real-time reverse transicription PCR method) |
 |
106-0057-01 | SARS-2-CoV(Covid-19)/Influenza A Virus / Influenza B Virus/Respiratory Syncytial Virus Diagnostic Kit (PCR-Fluorescence) | |
| Respiratory Infectious Diseases | 106-0038-01 | Flu A/FluA H1/FluA H3 /FluB Victoria/FluB Yamagata Diagnostic Kit (Real-time PCR) | |
| 106-0040-01 | RSV A/RSV B Diagnostic Kit (Real-time PCR) | ||
 |
106-0056-01 | Diagnostic Kit for Mycobacterium Tuberculosis(MTB)complex DNA and Rifampicin resistance Mycobacterium tuberculos (PCR-Fluorescence) | |
 |
106-0052-01 | Influenza A Virus / Influenza B Virus/Respiratory Syncytial Virus Diagnostic Kit (PCR-Fluorescence) | |
| 106-0069-01 | MP/CP/AdV Diagnostic Kit (Real-time PCR) | ||
 |
106-0055-01 | Streptococcus Pneumoniae/ Klebsiella Pneumoniae/ Legionella Pneumophila/ Haemophilus influenzae/ Bordetella Pertussis Diagnostic Kit (PCR-Fluorescence) |
|
| Intestinal Infectious Diseases |  |
106-0054-01 | Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit(PCR-Fluorescence) |
 |
106-0053-01 | Coxsackievirus A16/Enterovirus 71/Enterovirus RNA Diagnostic Kit(PCR-Fluorescence) | |
| Sexual Infectious Diseases | 106-0068-01 | Chlamydia trachomatis/Niesseria gonorrhoeae/Ureaplasma urealyticum Diagnostic Kit (PCR-Fluorescence) | |
| Gynecologic Oncology |  |
106-0066-01 | Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV) (PCR fluorescence probe method) |
| 106-0064-01 | Nucleic acid detection kit for 16&18 human papillomavirus (HPV) genotypes (PCR fluorescence probe method) | ||
| Arbo Infectious Diseases | 106-0044-01 | DENV/CHIKV/ZIKV Diagnostic Kit (Real-time PCR) | |
| Universal kit |  |
106-0051-01 | Nucleic Acid Extraction and Purification Kit |
Linear Results
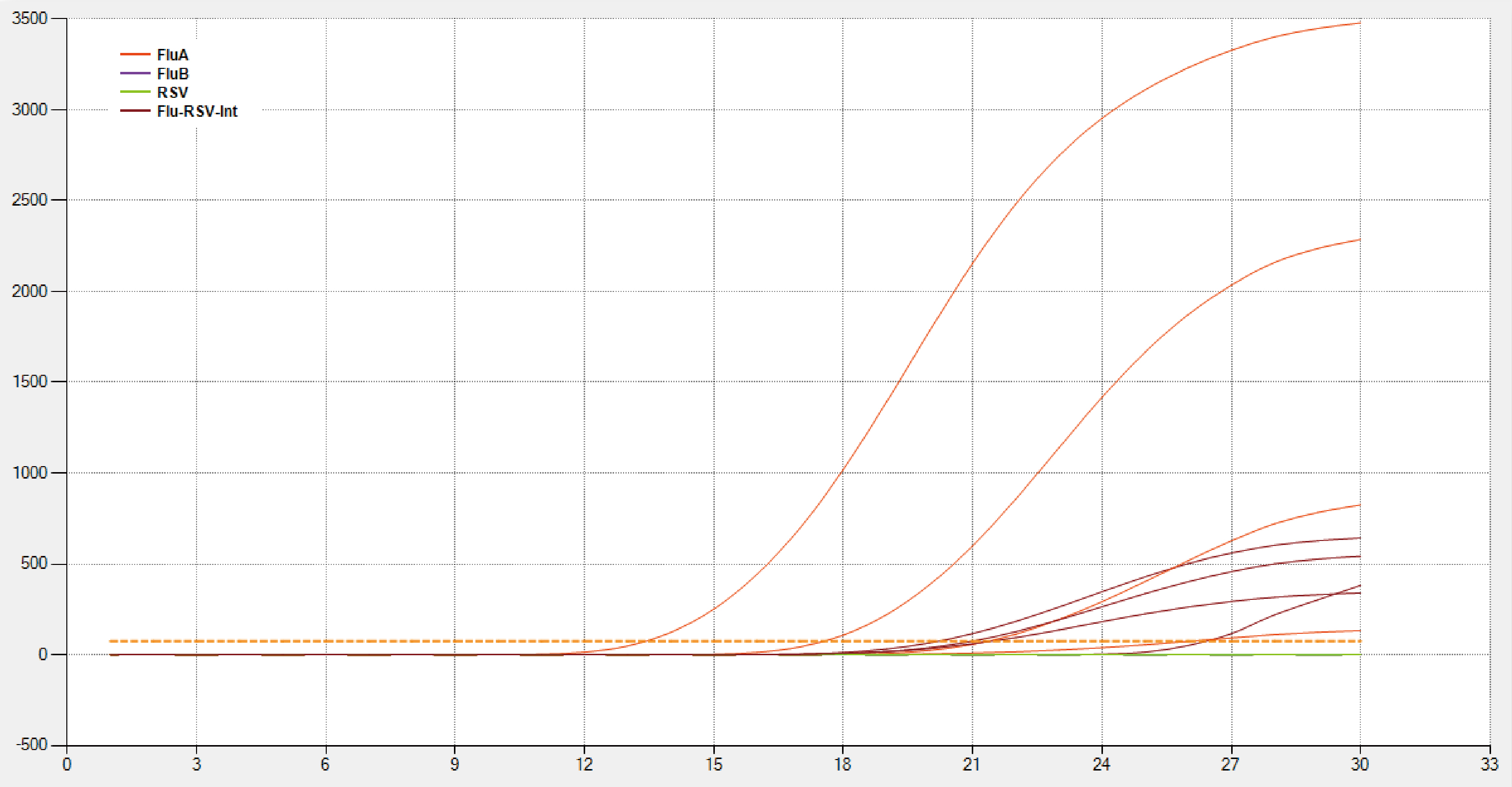
These are the linear results of influenza A viruses (seasonal H1N1) detected from the Detection Limit National reference panel for influenza A/B viruses nucleic acids analysis reagent. The concentrations are 2.5×10e³(TCID50/L), 2.5×10e² (TCID50/L), 2.5×10 (TCID50/L), 2.5 (TCID50/L).
Accuracy
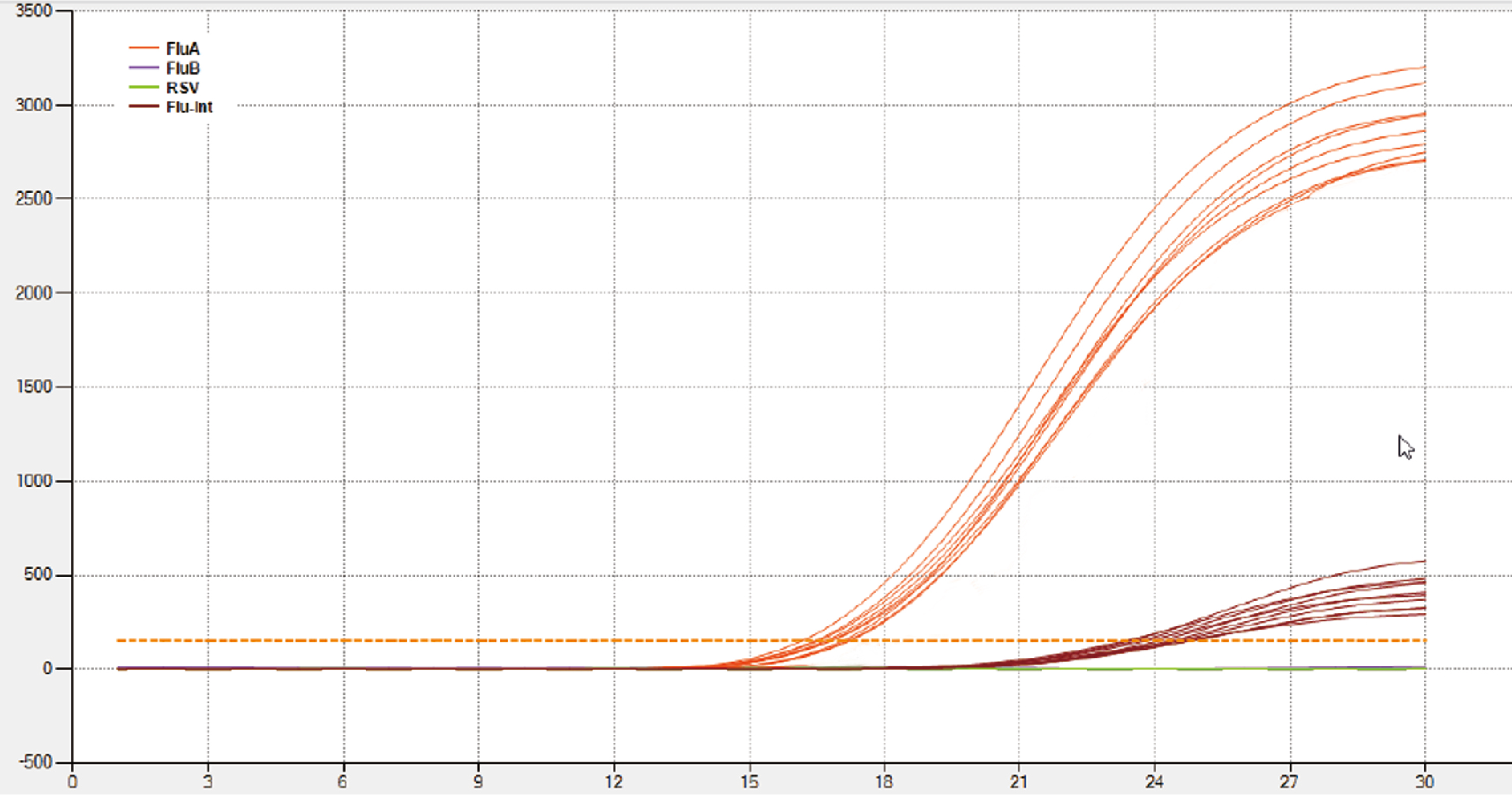
These are the accurate results of influenza A viruses (seasonal H3N2) detected from the Accurate National reference panel for influenza A/B viruses nucleic acids analysis reagent, diluted by 1:10, and the concentration is 6.0×10³ (TCID50/L).
Application
The Galaxy series is an intelligent, fully enclosed, and integrated system, enabling users to use it in different scenarios. It has high sensitivity and qPCR specificity. At the same time, it ensures easy use in the simplest experimental environments, eliminates PCR laboratory contamination and guarantees biosafety.

