Gynecologic Oncology
Cervical cancer is a Global Public Health Problem
In 2020, an estimated 604,000 women were diagnosed with cervical cancer worldwide, and about 342,000 women died from the disease. In November 2020, the WHO launched their global strategy to accelerate the elimination of cervical cancer with the goal of achieving the following by 2030: a 90% human papillomavirus (HPV) vaccination coverage of eligible women, a 70% screening coverage with a high-performance test, and 90% of women with a positive screening test result or a cervical lesion(s) treated appropriately [1].
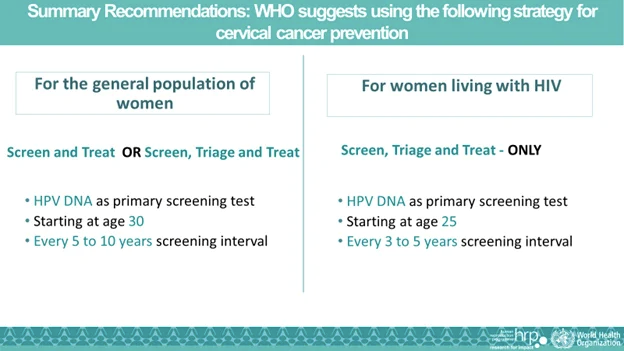
New Recommendation from the WHO for HPV DNA Primary Screening Test
On July 6, 2021, the World Health Organization (WHO) issued their 2nd edition guidelines for the screening and treatment of cervical pre-cancerous lesions for cervical cancer prevention. The guideline recommended HPV DNA as the primary screening test to promote cervical cancer prevention and save more lives [2].
Igenesis’s POCT molecular diagnostic platform for HPV DNA detection has broken through previous PCR lab restrictions. Using classic magnetic bead extraction and fluorescent PCR technology for accurate HPV DNA testing in a broader range of scenarios will improve the efficiency of cervical cancer screening and contribute to the prevention and mitigation of cervical cancer.