On December 19, 2023, Igenesis announced that its independently developed Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV) (PCR fluorescence probe method) has been approved by the Thai Ministry of Public Health after strict review. The reagent has obtained the TFDA registration certification of medical devices in Thailand, which qualifies it to enter the Thai medical device market and contribute to the local medical and health construction industry. This is another piece of good news for Igenesis, following the Thai medical device registration certificate obtained by its Automated Fully Enclosed qPCR System--Galaxy Nano and Galaxy Lite, TB Nucleic Acid Detection Reagent and Nucleic Acid Extraction and Purification Kit.
According to the World Health Organization (WHO), cervical cancer is the third most common cancer type among women worldwide, with an incidence rate only lower than lung and breast cancer. Approximately 600,000 women are diagnosed with cervical cancer every year globally, and about 340,000 of them die from this disease.
On November 17, 2020, the World Health Organization (WHO) launched the Global Strategy to Accelerate the Elimination of Cervical Cancer, emphasizing the importance of HPV testing and screening. On July 6, 2021, WHO updated and released the “Preventing Cervical Cancer: WHO Guidelines for Screening and Treatment of Precancerous Lesions of the Cervix", which recommends HPV DNA testing as the preferred screening method for cervical cancer.
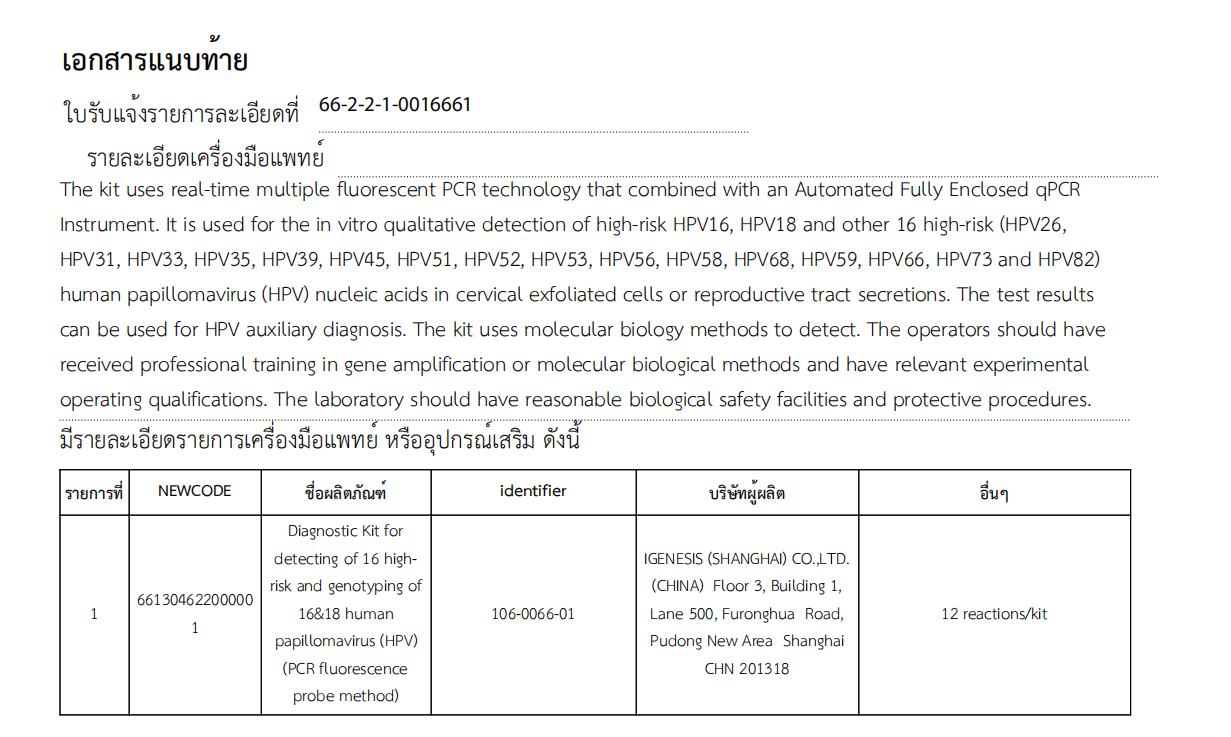
Igenesis’s Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV) (PCR fluorescence probe method) can be used for qualitative detection of high-risk 16 types (HPV16), high-risk 18 types (HPV18), and other 16 high-risk types (HPV26, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV53, HPV56, HPV58, HPV68, HPV59, HPV66, HPV73, and HPV82) of human papillomavirus in vitro from cervical exfoliated cells or genital secretions. The test results can be used for HPV-assisted diagnosis, improve the efficiency of cervical cancer screening, and effectively help prevent and control cervical cancer.
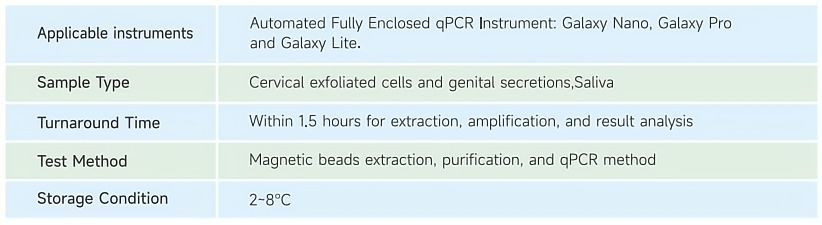
The HPV nucleic acid detection kit, when used in conjunction with the Galaxy Automated Fully Enclosed qPCR System, can complete the entire process of nucleic acid extraction, purification, and fluorescence PCR amplification in only 3 steps. The process is fully automatic and enclosed, and the results are accurate and reliable.

☑ Accurate results: CV value ≤5%, coincidence rate is 100%;
☑ Easy to operate: nucleic acid extraction and amplification analysis are fully automated, avoiding human errors, with good result repeatability, capable of single-sample detection, and can be tested anytime;
☑ Safe to use: Fully enclosed iCassette, Prevent pollution, Ensure biosafety
☑ Solid material transfer+Microfluidic technology;
☑ Preloading reagent+Patented freeze-drying technology;
☑ No need for frozen transport.

Igenesis focuses on real-time molecular diagnostic technology. Until October 2023, igenesis has obtained CE mark for 19 reagents and instruments,overseas business is distributed globally, such as Italy, Chile, Malaysia, Spain, Denmark, Hong Kong, China and Europe, South America, Southeast Asia and other regions. In the future, igenesis will continue to explore the infinite possibilities of gene detection with the enterprise philosophy of “integrity, excellence, innovation, and win-win”, and provide customers with safe, fast, convenient, accurate molecular diagnostic integrated products, services, and solutions.
Post time: Jan-16-2024
