Recently,Igenesis has achieved another milestone in its overseas registration process. Diagnostic Kit for Mycobacterium Tuberculosis(MTB) complex DNA and Rifampicin resistance Mycobacterium tuberculosis (PCR-Fluorescence), has obtained Thailand's medical device TFDA registration and certification, and qualified to enter Thailand's medical device market.

Diagnostic Kit for Mycobacterium Tuberculosis(MTB) complex DNA and Rifampicin resistance Mycobacterium tuberculosis (PCR-Fluorescence), coupled with Igenesis’s Fully Automated Closed qPCR System, can fully automated and enclosed complete the entire process of nucleic acid extraction, purification, and fluorescence PCR amplification in four simple steps. This kit is suitable for auxiliary detection of Mycobacterium tuberculosis infection and rifampicin resistance mutations.

Parameters
|
Applicable instrument |
Automated Fully Enclosed qPCR Instryment: Galaxy Pro,Galaxy Lite,Galaxy Nano |
|
Sample Type |
Sputum |
|
Turnaround Time |
Within 2 hours for extraction, amplification and result analysis |
|
Test Method |
Magnetic beads extraction, purification and qPCR method |
|
Target |
TB:IS6110, RIF:ropB 81bp |
|
Storage Condition |
2~8℃ |
Advantage
High-efficiency testing
With the first seven-color fluorescence system in China, to achieve multiple targets for tuberculosis and rifampicin resistance in one tube. It improves the efficiency of tuberculosis detection.
Easy to use
Fully automatic, sample in, result out.
Safe to use
Fully enclosed iCassette prevents internal or external contamination and guarantees biosafety.
Accurate results
Ultrasonic lysis, magnetic bead extraction, qPCR analysis, and internal control monitor the entire process.
Workflow
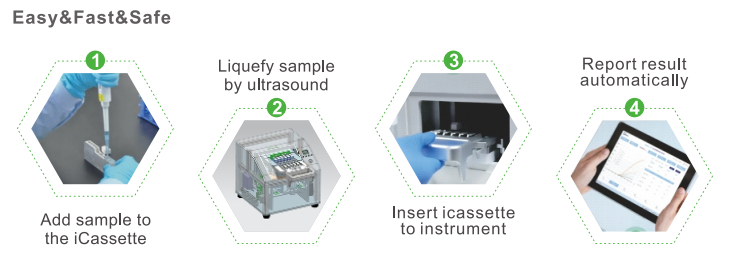
Clinical significance
⚫ To improving the positive rate of tuberculosis
⚫ To guiding rational and effective drug use in tuberculosis patients
⚫ Assisting clinical rapid designation and effective decision
Igenesis focuses on real-time molecular diagnostic technology. Until October 2023, igenesis has obtained CE mark for 19 reagents and instruments,overseas business is distributed globally, such as Italy, Chile, Malaysia, Spain, Denmark, Hong Kong, China and Europe, South America, Southeast Asia and other regions. In the future, igenesis will continue to explore the infinite possibilities of gene detection with the enterprise philosophy of “integrity, excellence, innovation, and win-win”, providing customers with safe, fast, convenient, and accurate POCT solutions for our customers.
Post time: Oct-13-2023
