Igenesis, a renowned medical diagnostics company, has garnered international acclaim for its three proprietary test kits. These kits, namely the Coxsackievirus A16/Enterovirus 71/Enterovirus RNA Diagnostic Kit (PCR-Fluorescence), the Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit (PCR-Fluorescence), and the Chlamydia trachomatis/Neisseria gonorrhoeae/Ureaplasma urealyticum Diagnostic Kit (PCR-Fluorescence), have successfully navigated the stringent approval process of the Thai Ministry of Public Health. Consequently, they have secured registration certification from the Thai FDA, marking Igenesis's official entry into the Thai medical device market. With a commitment to enhancing public healthcare standards in Thailand, Igenesis aims to equip local healthcare institutions with cutting-edge disease prevention and control solutions.
PCR molecular diagnosis of Hand foot and mouth Disease (HFMD)
Hand, foot, and mouth disease (HFMD) is a prevalent childhood infectious illness primarily caused by enterovirus infections, predominantly affecting children under 5 years of age. In China, the incidence of HFMD ranges from 37.01 to 205.06 per 100,000 individuals, with reported fatality rates varying from 6.46 to 51.00 per 100,000. The key pathogenic serotypes linked to HFMD encompass Coxsackievirus (CA) A groups 4–7, 9, 10, 16, and B groups 1–3, 5, along with Echovirus serotypes and Enterovirus 71 (EV71). Notably, CA16 and EV71 are the most prevalent serotypes, with EV71 being associated with the most severe cases and fatalities.
It is crucial to highlight that there is no cross-immunity between different enterovirus types, indicating that a child who has experienced HFMD once remains susceptible to a potential second infection. PCR molecular detection stands out for its high specificity in distinguishing HFMD from other conditions such as rubella and Herpetic pharyngobuccinitis, significantly boosting the efficiency of rapid diagnosis. Moreover, PCR molecular detection exhibits exceptional sensitivity during the infection's window period, providing invaluable information for clinical treatment strategies and epidemic prevention efforts.
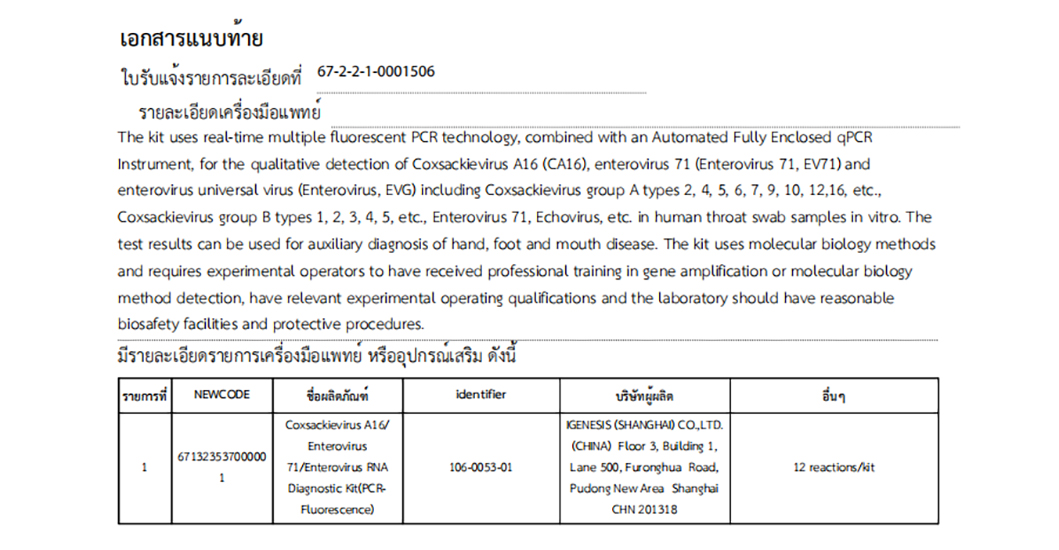
Coxsackievirus A16/Enterovirus 71/Enterovirus RNA Diagnostic Kit(PCR-Fluorescence) 3-plexes Detection
For the qualitative detection of Coxsackievirus A16 (CA16), enterovirus 71 (Enterovirus 71, EV71) and enterovirus universal virus (Enterovirus, EVG) including Coxsackievirus group A types 2, 4, 5, 6, 7, 9, 10, 12,16, etc., Coxsackievirus group B types 1, 2, 3, 4, 5, etc., Enterovirus 71, Echovirus, etc.
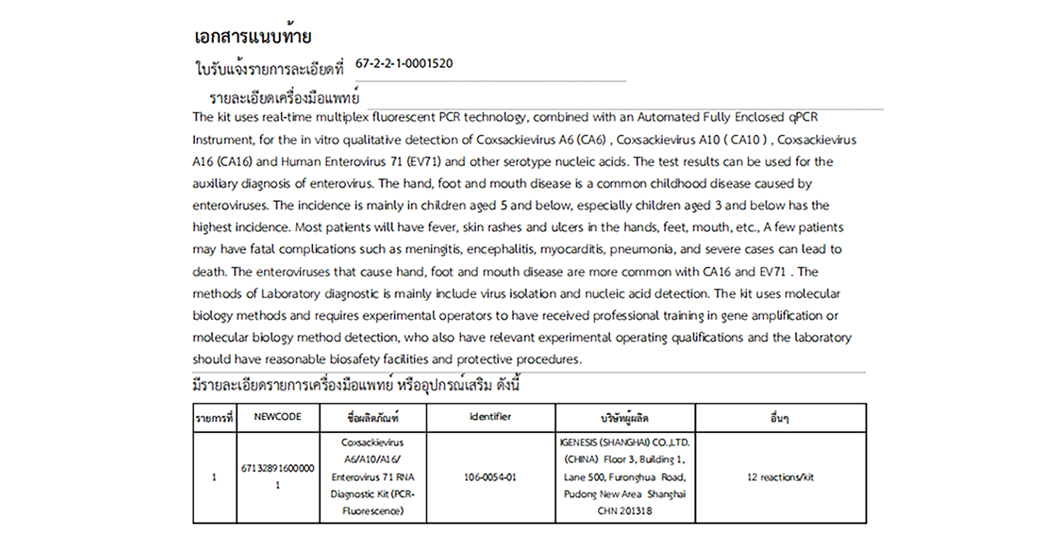
Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit(PCR-Fluorescence) 4-plexes Detection
For the in vitro qualitative detection of Coxsackievirus A6(CA6), Coxsackievirus A10(CA10), Coxsackievirus A16(CA16) and Human Enterovirus 71(EV71) and other serotype nucleic acids.
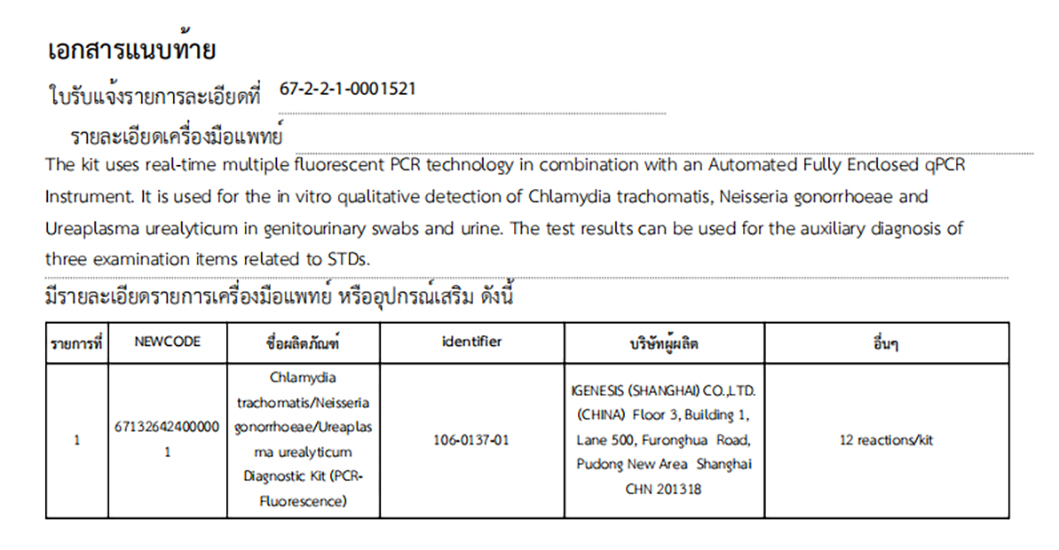
Nucleic Acid Detection for Chlamydia trachomatis, Gonorrhoeae, and Ureaplasma Urealyticum (PCR-Fluorescent Probe Method)
Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Ureaplasma urealyticum (UU) pose a wide range of hazards, affecting multiple organs including the eyes, reproductive tract, and rectum. While many infected individuals experience subtle symptoms, the infection can persist, resulting in a prolonged illness, long-term health issues, and an elevated risk of human transmission.
The co-infection rate of CT/NG/UU is remarkably high. Infection with a single pathogen can easily lead to cross-mixed infections with other pathogens, resulting in complications.
Multiplex detection of CT/NG/UU can effectively identify infected patients, distinguish co-infections, enhance the efficiency of screening, diagnosis, and treatment for reproductive tract infections, and facilitate early diagnosis and management.
The Centers for Disease Control and Prevention (CDC) recommends molecular diagnosis as the most sensitive and preferred test for detecting Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum.
The Chlamydia trachomatis/Neisseria gonorrhoeae/Ureaplasma urealyticum Diagnostic Kit (PCR-Fluorescence) by Igenesis (Shanghai) Co., Ltd. is an in vitro qualitative test designed to detect Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum DNA in cervical swabs from females and urethral swabs from males. These results serve as a valuable adjunct diagnostic tool for identifying sexually transmitted infections.
CA16/EV71/EVG Diagnostic Kit, CA6/CA10/CA16/EV71 Diagnostic Kit , and the CT/NG/UU Diagnostic Kit, when utilized in conjunction with the Galaxy fully automatic nucleic acid detection and analysis platform, streamline the process into just 3 simple steps. This comprehensive system carries out the entire procedure of nucleic acid extraction, purification, and fluorescent PCR amplification in a fully automated and hermetically sealed manner, ensuring precise and dependable results.

Test Process
Receive qPCR test results in just 3 steps

Igenesis specializes in point-of-care molecular diagnostic technology.By April 2024, the company has achieved EU CE certification for 21 products, expanding its global presence to include Italy, Chile, Malaysia, Spain, Denmark, Hong Kong, China, and various other regions across Europe, South America, and Southeast Asia.
Looking ahead, Igenesis remains committed to its core values of integrity, excellence, innovation, and fostering mutually beneficial partnerships.
The company will persist in venturing into new frontiers of genetic testing, delivering customers with holistic molecular diagnostic solutions that are not only safe, but also swift, convenient, and highly accurate.
Igenesis has obtained Thailand’s TFDA certified product catalog
| Catalog | Product | Specification |
| 101-0022-02 | Automated Fully Enclosed qPCR Instrument Galaxy Nano | Unit |
| 101-0038-01 | Automated Fully Enclosed qPCR Instrument Galaxy Lite | Unit |
| 106-0051-01 | Nucleic Acid Extraction and Purification Kit (One in one) | 12 Test/kit |
| 106-0119-01 | Nucleic Acid Extraction and Purification Kit(Three in one) | 6 Test/kit |
| 106-0056-01 | Diagnostic Kit for Mycobacterium Tuberculosis(MTB)complex DNA and Rifampicin resistance Mycobacterium tuberculosis (PCR-Fluorescence) | 12 Test/kit |
| 106-0066-01 | Diagnostic Kit for detecting of 16 high-risk and genotyping of 16&18 human papillomavirus (HPV)(PCR fluorescence probe method) | 12 Test/kit |
| 106-0053-01 | Coxsackievirus A16/ Enterovirus 71/Enterovirus RNA Diagnostic Kit(PCR-Fluorescence) | 12 Test/kit |
| 106-0054-01 | Coxsackievirus A6/A10/A16/Enterovirus 71 RNA Diagnostic Kit(PCR-Fluorescence) | 12 Test/kit |
| 106-0137-01 | Chlamydia trachomatis/Neisseria gonorrhoeae/Ureaplasma urealyticum Diagnostic Kit (PCR-Fluorescence) | 12 Test/kit |
Post time: Apr-26-2024
