Recently, multiple PCR nucleic acid detection kits independently developed by Igenesis have successfully obtained medical device registration certificates issued by the Moroccan health authority. This important certification not only signifies international authoritative recognition of Igenesis’ product quality, performance, and safety but also lays a solid foundation for the company to further expand into North African and global markets.
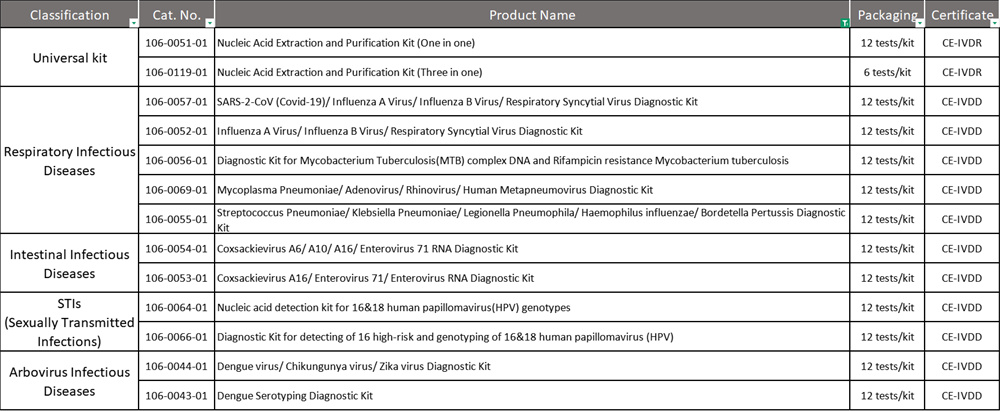
The certified products cover diverse nucleic acid detection categories—respiratory pathogens, HPV, tropical viruses, bacterial pathogens, and tuberculosis drug resistance testing—addressing clinical needs in respiratory infections, tropical diseases, female health management, and tuberculosis control. Their successful registration will provide reliable molecular diagnostic solutions for local disease screening, clinical diagnosis, and public health initiatives in Morocco.
This achievement not only reflects the Moroccan medical device authority’s recognition of Igenesis’ R&D capabilities and quality system but also demonstrates the company’s unwavering commitment to innovation in point-of-care molecular diagnostics (POCT) and global market expansion.
Moving forward, guided by the corporate philosophy of ”Integrity, Excellence, Innovation, and Mutual Benefit”, Igenesis will continue optimizing its product portfolio and developing diversified testing solutions. The company remains dedicated to delivering efficient, convenient, and reliable comprehensive molecular POCT solutions worldwide—actively supporting global public health initiatives and safeguarding human well-being.
Certified Products List :
Post time: May-29-2025